Introduction
The Impact of mRNA COVID-19 Vaccines
One of the most poignant experiences I had with COVID-19 occurred during a cancer support group meeting. It was at the height of the pandemic, before any vaccines were available. In a tear-filled episode, a patient navigating the difficulties of terminal cancer shared that he had lost his husband and primary caregiver to COVID-19. This story, like many others, underscores the profound impact COVID-19 vaccines have had on people’s lives. The rapid development and high efficacy of these vaccines, which brought relief and hope to millions, can largely be attributed to the innovative technology of mRNA vaccines.
The introduction of mRNA vaccines has reshaped global health, providing an advanced method for preventing infectious diseases. The success of mRNA vaccines in combating the COVID-19 pandemic has shown their potential to address a wide range of health challenges. Unlike traditional vaccines, which use weakened or inactivated pathogens, mRNA vaccines employ a novel method that has rapidly changed immunization practices.
This new technology uses mRNA to instruct cells to produce a protein that triggers an immune response, providing protection against the virus. This approach accelerates the vaccine development process and allows for quick adaptation to emerging viral variants. The pioneering work that led to the development of mRNA vaccines was recognized with the 2023 Nobel Prize in Physiology or Medicine. This honor indicates the significant scientific advancements and collaborative efforts that have made mRNA vaccines a reality.
Understanding mRNA Vaccines
In this blog post, I’ll give an overview of the science behind mRNA vaccines, and the significant benefits they offer. I’ll cover the historical context of vaccine development, providing a comparison with traditional vaccines.By understanding these aspects, we can appreciate the substantial impact of mRNA vaccine technology on global health.
Then, I’ll discuss the challenges and limitations associated with mRNA vaccines and examine their future prospects. The potential for mRNA vaccines to address many infectious beyond COVID-19 is immense. Moreover, as I will explain, the technology behind these vaccines holds promise for delivering other types of biologics, potentially offering treatments for an even broader spectrum of health conditions.
Overview and Comparison of mRNA Vaccines with Traditional Vaccines
Traditional Vaccines
There are several established methods to produce vaccines, each utilizing different approaches to stimulate the immune system. Traditional vaccines include inactivated vaccines; live attenuated vaccines; as well as a third class known as variously as subunit, recombinant, polysaccharide, conjugate, and toxoid vaccines.
Inactivated vaccines are created from viruses or bacteria that have been killed. These pathogens cannot cause disease but still trigger an immune response. Examples include the polio vaccine and some flu vaccines. While effective, inactivated vaccines often require multiple doses to achieve long-lasting immunity.
Live attenuated vaccines use live viruses or bacteria that have been weakened so they cannot cause serious illness in healthy people. They provoke a strong and lasting immune response and are used in vaccines like MMR (measles, mumps, rubella) and varicella (chickenpox). However, these vaccines can pose risks to immunocompromised individuals due to the use of live pathogens.
Subunit, recombinant, polysaccharide, and conjugate vaccines focus on specific pieces of the pathogen, such as its protein, sugar, or capsid. This targeted approach minimizes side effects and directs the immune response effectively. Examples include the Hepatitis B vaccine (recombinant), HPV vaccine (subunit), and pneumococcal vaccine (conjugate). These vaccines do not use the entire pathogen, reducing the risk of adverse reactions.
Toxoid vaccines are made from toxins produced by the pathogen that have been inactivated. These vaccines help the immune system recognize and neutralize the toxins. Tetanus and diphtheria vaccines are common examples.
mRNA vaccines
mRNA vaccines represent a significant advancement in vaccine technology. Unlike traditional vaccines, mRNA vaccines use messenger RNA to instruct cells to produce a protein that triggers immunity. This approach was used successfully in the Pfizer-BioNTech and Moderna COVID-19 vaccines. mRNA vaccines can be developed rapidly since synthesizing mRNA is faster than culturing live viruses.
Additionally, mRNA vaccines are highly adaptable, allowing for quick modifications to address emerging viral variants. Despite challenges like cold storage requirements and managing immune responses, mRNA vaccines offer a modern and efficient alternative, with potential applications beyond infectious diseases, including delivering biologics and treating various conditions. Overall, while traditional vaccines have been crucial in controlling numerous diseases, mRNA vaccines provide a promising future for global health with their rapid development and adaptability.
Mechanism of Action of mRNA Vaccines
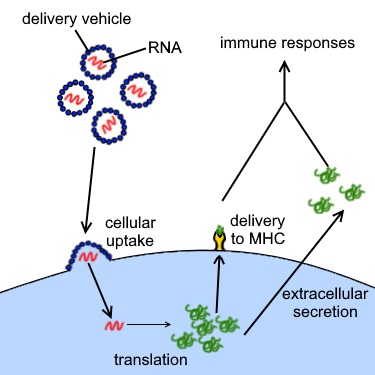
mRNA vaccines work by introducing mRNA into the body that encodes a specific viral protein. Here’s how the process works at the molecular level:
- Introduction of mRNA: When an mRNA vaccine is administered, the mRNA is encapsulated in lipid nanoparticles to protect it and facilitate its entry into human cells.
- Translation into Protein: Once inside the cells, the mRNA is translated by the cell’s ribosomes into the viral protein. This protein is usually a harmless piece of the virus, such as the spike protein of the SARS-CoV-2 virus that causes COVID-19.
- Immune System Activation: The newly produced viral proteins are then displayed on the surface of the cells. The immune system recognizes these proteins as foreign and mounts an immune response. This response includes the production of antibodies and activation of T-cells, which are crucial for fighting off the actual virus if the body is exposed to it in the future.
- Memory Formation: The immune system also creates memory cells that remember the viral protein. This ensures a rapid and robust response if the body encounters the virus again, providing long-term protection.
This mechanism of action allows mRNA vaccines to be developed more quickly than traditional vaccines, which require the cultivation and inactivation or attenuation of the virus. Additionally, mRNA vaccines can be easily adapted to target new viral variants by simply altering the mRNA sequence. This adaptability is a significant advantage in responding to emerging infectious diseases and pandemics.
Types of Vaccines
| Type of Vaccine | Description | Examples |
| mRNA Vaccines | Use messenger RNA to instruct cells to produce a protein that triggers an immune response without using a live pathogen. | Pfizer-BioNTech COVID-19 vaccine, Moderna COVID-19 vaccine |
| Inactivated Vaccines | Made from viruses or bacteria that have been killed. These pathogens cannot cause disease but still stimulate an immune response. | Polio vaccine, some flu vaccines |
| Live Attenuated Vaccines | Use live viruses or bacteria that have been weakened so they cannot cause serious illness in healthy people. They provoke a strong and lasting immune response. | Measles, mumps, rubella (MMR) vaccine, varicella (chickenpox) vaccine |
| Subunit, Recombinant, Polysaccharide, and Conjugate Vaccines | Use specific pieces of the pathogen, such as its protein, sugar, or capsid. This targeted approach minimizes side effects and focuses the immune response. | Hepatitis B vaccine (recombinant), HPV vaccine (subunit), pneumococcal vaccine (conjugate) |
| Toxoid Vaccines | Made from toxins produced by the pathogen that have been inactivated. They help the immune system recognize and neutralize the toxins. | Tetanus, diphtheria vaccines |
Development of mRNA Vaccines
Historical Context
The development of mRNA vaccines is a milestone in immunization, rooted in decades of scientific research and innovation. Traditional vaccine development began with Edward Jenner’s smallpox vaccine in the late 18th century. Louis Pasteur’s work on rabies and anthrax vaccines in the 19th century further advanced the field. These early efforts laid the foundation for modern vaccinology. This led to various vaccines that significantly reduced the prevalence of many infectious diseases.
Early Research and Key Breakthroughs
The concept of using mRNA for vaccination emerged in the 1990s, with key contributions from researchers such as Katalin Karikó and Drew Weissman. Karikó, working at the University of Pennsylvania, focused on mRNA technology and its potential for medical applications. In collaboration with Weissman, the team discovered how to modify mRNA to reduce its inflammatory response, making it a viable candidate for vaccines. In their pivotal work published in 2005, they demonstrated that modified mRNA could be safely administered to cells to produce proteins, paving the way for mRNA-based therapeutics and vaccines.
Major Milestones in the Development of mRNA Vaccines
Several key milestones have marked mRNA vaccine development. In 2013, Moderna, a biotechnology company, began exploring mRNA-based vaccines and therapeutics, leveraging the foundational research of Karikó and Weissman. In parallel, BioNTech, a German biotechnology company led by Uğur Şahin and Özlem Türeci, also invested in mRNA technology for cancer immunotherapy and infectious disease vaccines. These companies, along with CureVac, another pioneering firm in mRNA technology, made significant strides in developing mRNA platforms that could be rapidly adapted for various diseases.
The COVID-19 pandemic accelerated the development of mRNA vaccines, with Moderna and BioNTech, in partnership with Pfizer, leading. The rapid development of the Pfizer-BioNTech and Moderna vaccines demonstrated mRNA technology’s potential to respond quickly to health crises. These vaccines showed high efficacy rates in clinical trials and were authorized for emergency use within a year. This was an unprecedented achievement in vaccine history.
Nobel Prize Recognition
The Nobel Prize Committee recognized the groundbreaking work that led to the development of mRNA vaccines with the 2023 Nobel Prize in Physiology or Medicine. They awarded this prestigious honor to Katalin Karikó and Drew Weissman for their pioneering research on mRNA technology, which paved the way for the creation of mRNA vaccines. Their contributions have both revolutionized the field of vaccinology, and opened new avenues for treating a wide range of diseases.
Benefits of mRNA Vaccines
Efficacy of mRNA Vaccines
Both mRNA and traditional vaccines are highly effective. For example, receiving two or three doses of the COVID-19 vaccine reduces the risk of needing mechanical ventilation or dying by 90%. Similarly, the smallpox vaccine is 95% effective, and the inactivated polio vaccine is 90% effective with two doses and 99-100% effective with three doses.
However, the effectiveness of traditional vaccines can vary. For instance, the flu vaccine reduces the risk of severe flu by 40-60% because many different flu viruses constantly mutate, requiring annual updates. mRNA vaccines exhibit high efficacy against COVID-19, but researchers still need to fully understand their effectiveness against other viruses, as they have only extensively tested one virus so far. Despite variability, doctors still recommend vaccines like the annual flu shot because they can save lives.
Speed of Development of mRNA Vaccines
One of the most impressive benefits of mRNA vaccines is their rapid development timeline. Traditional vaccines often take years to develop because of the need to grow and inactivate pathogens or isolate specific proteins. In contrast, researchers can design and produce mRNA vaccines within weeks once they identify the virus’s genetic sequence. This quick turnaround was vital during the COVID-19 pandemic, allowing for the swift creation and deployment of effective vaccines. I consider this ability to respond rapidly to emerging infectious diseases one of the most exciting aspects of mRNA vaccine technology.
Adaptability of mRNA Vaccines
mRNA vaccines are incredibly adaptable. Scientists can quickly modify them to address new virus variants, which is crucial in a constantly evolving world. For example, when new variants of the coronavirus emerged, scientists could adjust the mRNA sequences in the vaccines to better match these variants. This adaptability ensures that mRNA vaccines can remain effective even as viruses change. I find this flexibility particularly promising, as it means we can stay one step ahead of potential pandemics.
Safety Profile
The safety profile of mRNA vaccines is another significant benefit. Clinical trials and real-world data have shown that these vaccines are generally safe and well-tolerated. Common side effects are usually mild and short-lived, such as sore arms, fatigue, and mild fevers. Serious side effects are rare.
The rigorous testing and monitoring of mRNA vaccines have provided a strong safety record, which is reassuring for those who might be hesitant. Because mRNA vaccines do not contain viruses or any other microbes, they cannot give a person an infection.
Challenges and Limitations of mRNA Vaccines
Storage and Distribution of mRNA Vaccines
One of the biggest challenges with mRNA vaccines is their storage and distribution. These vaccines need to be kept at ultra-cold temperatures to remain effective. For instance, the Pfizer-BioNTech COVID-19 vaccine requires storage at around -70°C (-94°F), which is much colder than standard freezers. This requirement poses significant logistical hurdles, especially in regions without advanced cold chain infrastructure.
Manufacturing Complexity of mRNA Vaccines
Manufacturing mRNA vaccines at scale is another significant challenge. The process involves sophisticated technology and stringent conditions to produce high-quality mRNA and ensure its stability. This complexity means that only a few facilities worldwide are currently equipped to produce these vaccines at the necessary scale. Expanding manufacturing capabilities and streamlining production processes are crucial steps in meeting global demand.
Public Hesitancy toward mRNA Vaccines
Public hesitancy and misinformation are major barriers to the widespread adoption of mRNA vaccines. However, it’s important to note that conventional vaccines also suffer from public hesitancy. Despite robust safety data and high efficacy rates, some people remain skeptical about these new vaccines, as well as traditional ones. Misinformation spread through social media and other channels can amplify fears and doubts for both types of vaccines. Providing clear, accurate information and addressing concerns directly can help build public trust and increase vaccination rates. By fostering a better understanding of vaccine science, we can overcome hesitancy and ensure broader public health protection.
Using mRNA Vaccine Technology to Treat Other Diseases
The development and deployment of mRNA vaccines significantly impacted the Covid pandemic. They both reduced the spread of the virus and prevented severe illness and death. mRNA vaccine technology has shown incredible potential beyond infectious diseases, however. This technology’s versatility and adaptability open up new possibilities for treating various health conditions. In this section, I’ll enumerate some of the other diseases this technology can potentially be applied to.
Infectious Diseases Beyond COVID-19
The process of developing mRNA vaccines for other infectious diseases is similar to that used for COVID-19. Researchers are working on creating mRNA vaccines for diseases like influenza, HIV, and Zika. These efforts aim to leverage the speed and precision of mRNA technology to improve response times and effectiveness. By using mRNA vaccines, we could potentially respond more quickly to emerging infectious diseases and improve overall public health outcomes.
Autoimmune Diseases
mRNA vaccines also show promise in treating autoimmune diseases by modulating the immune system. For example, studies are exploring how mRNA vaccines can help treat conditions like multiple sclerosis and rheumatoid arthritis. These vaccines could potentially provide targeted immune responses, reducing symptoms and improving the quality of life for patients.
Cancer Treatment
One of the most exciting applications of mRNA technology is in cancer treatment. Researchers can design mRNA vaccines to target and kill cancer cells by instructing the immune system to recognize and attack them. Ongoing studies and clinical trials are focusing on mRNA cancer vaccines, with promising early results. This approach offers the potential for more personalized and effective therapies.
Prospects of Using mRNA Technology for Protein Therapeutics
Advantages of Using mRNA as a Delivery Mechanism for Protein Therapeutics
Using mRNA as a delivery mechanism for protein therapeutics offers several advantages. First, researchers can design mRNA to produce any protein, making it highly versatile. This allows for the development of a wide range of therapies tailored to specific needs. Additionally, mRNA production is generally faster and more cost-effective than traditional protein production methods, which involve complex processes like cell culture and purification.
Second, mRNA therapies can be quickly modified in response to new information, similar to how mRNA vaccines are adapted for new virus strains. This adaptability makes mRNA an ideal platform for developing personalized medicine. Third, because mRNA is a temporary blueprint that cells use to produce proteins, there is less risk of long-term integration into the genome, which can be a concern with other genetic therapies.
Combination Therapies
Another exciting prospect is combining mRNA technology with other treatments. For example, doctors can use mRNA vaccines alongside traditional cancer therapies like chemotherapy and radiation to enhance their effectiveness. This combination approach could lead to more comprehensive treatment plans and improved patient outcomes.
Genetic Disorders
mRNA technology also holds potential for correcting genetic mutations. By delivering mRNA sequences that encode functional proteins, we could treat genetic disorders that currently have no cure. This approach offers hope for conditions like cystic fibrosis and muscular dystrophy, where traditional treatments have limited success.
Limitations of mRNA Technology for Protein Therapeutics
Technical Challenges
While the potential is vast, there are limitations to using mRNA technology for protein therapeutics. One major challenge is ensuring the stability of mRNA in the body. mRNA is inherently unstable and can degrade quickly, which poses a challenge for consistent therapeutic delivery. This issue is particularly relevant for biological therapies that require long-term or indefinite administration. Unlike vaccines, doctors may need to deliver protein therapeutics repeatedly over a longer period. Scientists are working on improving mRNA stability and delivery methods, but it remains a significant hurdle.
Regulatory Challenges
Developing new mRNA therapies involves navigating complex regulatory landscapes to ensure safety and efficacy. Regulatory bodies must adapt to mRNA technology, assessing stability, delivery efficiency, and long-term effects. This adaptation can slow down approval processes as frameworks evolve for mRNA therapeutics.
Despite these hurdles, the regulatory path for mRNA therapies might be less burdensome than for conventional biologics. The manufacture of conventional biologics is complex, requiring precise Good Manufacturing Practice (GMP) protocols and addressing issues like contamination and consistency. Any modification requires FDA reevaluation. In contrast, mRNA manufacturing is more straightforward and adaptable, allowing for quicker modifications and potentially more streamlined regulatory approval processes.
Cost and Accessibility
Cost and accessibility are also significant factors for all drugs. Producing mRNA therapies at scale and ensuring they are affordable for all patients is crucial. This requires investment in manufacturing infrastructure and policies that support equitable access to these treatments. However, RNA is cheaper and easier to produce than protein biologics. This efficiency reduces costs for patients and healthcare systems, enhancing accessibility. The simpler design and manufacturing processes for mRNA therapies also facilitate quicker scalability and distribution, promoting global health equity, especially in low- and middle-income countries.
A Synopsis of Traditional Vaccines vs. mRNA Vaccines
| Element | Traditional Vaccines | mRNA Vaccines |
| Definition | Use weakened, inactivated, or parts of a pathogen to stimulate an immune response. | Use messenger RNA (mRNA) to instruct cells to produce a protein that triggers an immune response. |
| Mechanism of Action | Introduce a form of the pathogen to train the immune system to recognize and combat it. | Introduce mRNA that encodes a viral protein, which cells produce, prompting an immune response. |
| Types | Inactivated, Live Attenuated, Subunit, Toxoid, Conjugate | mRNA-based |
| Production Process | Grow pathogen, inactivate or weaken it, purify, formulate into a vaccine. | Synthesize mRNA in the lab, encapsulate in lipid nanoparticles, formulate into a vaccine. |
| Development Time | Several years to decades. | Few months to a couple of years. |
| Adaptability | Limited; modifying vaccines for new strains is time-consuming. | Highly adaptable; can be quickly modified to target new strains. |
| Storage Requirements | Typically refrigerated, some require freezing. | Require ultra-cold storage temperatures. |
| Safety Profile | Extensively studied over decades; well-understood long-term effects. | New technology; safety profile is positive based on clinical trials, but long-term effects are less known. |
| Challenges | Long production times, challenges in culturing pathogens, less adaptable to new strains. | Ultra-cold storage, public hesitancy due to new technology, complex manufacturing processes. |
| Potential for Other Conditions | Generally limited to infectious diseases. | Broad potential applications, including cancer, genetic disorders, and autoimmune diseases. |
Conclusion
As we look toward the future, offer a glimpse into a new era of medical innovation. The swift development and deployment of these vaccines during the COVID-19 pandemic have demonstrated both the potential of mRNA technology, and the incredible power of scientific collaboration and human ingenuity. This achievement serves as a reminder that when faced with unprecedented challenges, we have the tools and creativity to overcome them.
Beyond their immediate applications in combating infectious diseases, mRNA vaccines open the door to a broader paradigm in medicine. Imagine a world where vaccines and therapies can be rapidly designed and tailored to individual needs, addressing conditions that were previously untreatable. This technology could revolutionize personalized medicine by permitting the specific tailoring treatments to the patients receiving them.
Moreover, the flexibility and scalability of mRNA vaccine technology hold promise for addressing global health disparities. By making vaccines easier and cheaper to produce, we can ensure that life-saving treatments reach even the most underserved populations. This democratization of healthcare could play a pivotal role in closing the gap between high-income and low-income countries, leading to more equitable health outcomes worldwide.
In the face of a constantly evolving health landscape, the story of mRNA vaccines is a beacon of hope. It shows that with determination, collaboration, and a commitment to scientific excellence, we can pave the way for a healthier, more resilient future.
Your Thoughts?
What do you think about the potential of RNA vaccine technology? Are you excited about the future possibilities of using mRNA technology to treat a wider range of diseases? We’d love to hear your opinions and experiences. Please share your thoughts in the comments at the bottom of the page!
Materials for Further Study
Books:
- “A Shot to Save the World: The Inside Story of the Life-or-Death Race for a COVID-19 Vaccine” by Gregory Zuckerman. This book offers a gripping account of the unprecedented global effort to develop COVID-19 vaccines. Gregory Zuckerman provides a behind-the-scenes look at the scientists, researchers, and companies that raced against time to create life-saving vaccines in record time.
- “The Vaccine: Inside the Race to Conquer the COVID-19 Pandemic” by Joe Miller, Uğur Şahin, and Özlem Türeci. Authored by the founders of BioNTech and a prominent journalist, this book delves into the remarkable journey of creating the first mRNA COVID-19 vaccine. It captures the scientific breakthroughs, challenges, and personal stories behind one of the most significant medical achievements of our time.
- “The Medical Revolution of Messenger RNA” by Fabrice Delaye. Cold Spring Harbor Press 2023. Fabrice Delaye explores the transformative potential of messenger RNA (mRNA) technology in this comprehensive book. Highlighting its applications beyond COVID-19, the author discusses how mRNA is set to revolutionize medicine and pave the way for new treatments for various diseases.
Research Papers and Articles:
- “mRNA vaccines in disease prevention and treatment” Zhang et al. (2023) Signal Transduction and Targeted Therapy. mRNA vaccines, highlighted by their success against COVID-19, show high efficacy, safety, and scalability, prompting broader applications despite design and delivery challenges. This review covers mRNA design, synthesis, delivery, and adjuvant technologies, analyzing their use in infectious diseases, cancers, and more, while discussing future advancements and challenges.
- “Nanotechnology-based mRNA vaccines” Chen et al. (2023). Nature Reviews Methods Primers. mRNA vaccines offer rapid, precise immune responses with advantages like fast production and high efficacy. Despite delivery challenges due to mRNA instability, nanotechnology enhances their potency. This Primer explores biomaterials and nanotechnology development for mRNA vaccines, focusing on design, formulation, applications, and future perspectives.
- “mRNA: Vaccine or Gene Therapy? The Safety Regulatory Issues” Banoun et al. 2023. Int J Mol. Sci. COVID-19 vaccines were rapidly approved without specific regulations, raising safety concerns. As mRNA vaccines should be classified as gene therapy products, thorough long-term studies on their safety, including potential adverse effects and horizontal transmission, are needed for future non-pandemic mRNA vaccines.
-
Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA Karikó, Katalin, et al. Immunity 23.2 (2005): 165-175 A seminal paper demonstrating that natural modifications to RNA, such as methylation, can prevent the immune system from overreacting to RNA. By incorporating these modified nucleosides into synthetic RNA, they were able to suppress immune activation. This breakthrough laid the foundation for the mRNA vaccine technology used today.
TED Talks
- “How mRNA medicine will change the world.” Melissa J. Moore 2022. Melissa J. Moore explains that mRNA medicine can treat diseases by instructing the body to produce essential proteins, with over 175 clinical trials showing its vast potential for conditions like cancer and autoimmune disorders, and its scalability for mass distribution.
- “Meet the scientist couple driving an mRNA vaccine revolution” Uğur Şahin and Özlem Türeci 2021. BioNTech cofounders Uğur Şahin and Özlem Türeci discuss with TED’s Chris Anderson how their mRNA research enabled the rapid development of the Pfizer-BioNTech COVID-19 vaccine and its future potential for vaccines and immunotherapy.
- How the COVID-19 vaccines were created so quickly – Kaitlyn Sadtler and Elizabeth Wayne 2021. Explains how mRNA vaccines use messenger RNA to instruct cells to produce proteins that trigger an immune response without causing infection, allowing for rapid, flexible, and less chemically intensive development compared to traditional vaccines.
Documentaries
- “How mRNA Vaccines Revolutionized Medicine” CNBC 2022. More than 30 biotech and pharmaceutical companies around the world raced to develop a safe Covid-19 vaccine. The process moved quickly with several vaccine candidates entering late-stage trials in a matter of months. Pfizer and Moderna used a promising new technology called messenger RNA.
- “Race for the Vaccine” BBC 2021. As news of the coronavirus broke around the globe, a small group of scientists jumped into action to tackle one of the greatest medical challenges of our time: to create a vaccine against a virus no one had ever seen before, and to do so in record time, during a deadly, global pandemic.
- “Revolution in medicine – BioNTech, mRNA and the Covid-19 vaccine” DW Documentary 2021. highlights the development of BioNTech’s COVID-19 vaccine using mRNA technology and the efforts of researchers Ugur Sahin and Ozlem Tureci. It also discusses the broader potential of mRNA technology for treating diseases like cancer and the significant impact of their vaccine on combating the pandemic.
- “The Messenger: A Story of mRNA” Inside Tomorrow Media 2023. How mRNA vaccine pioneers uncovered a complex history of discoveries, leading to groundbreaking mRNA technology that promises revolutionary cures for diseases like cancer and HIV. Initially aimed at gene therapy, their work earned the 2023 Nobel Prize in Medicine and is transforming the future of medicine.
Blogs
- Moderna Blog. Provides updates and insights on Moderna’s mRNA research, development processes, and advancements in mRNA therapeutics and vaccines.
- BioNTech Newsroom. Offers the latest news and information on BioNTech’s innovations, research milestones, and developments in mRNA technology.