Introduction
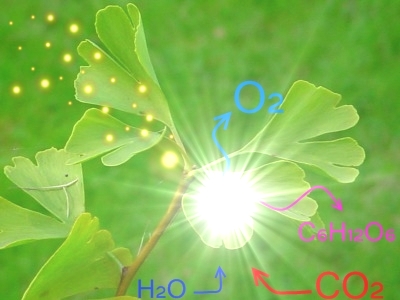
Imagine a world where we harness the power of the sun to create clean energy and reduce carbon dioxide levels, just like plants do. What if we could address climate change and the energy crisis by combining natural photosynthesis with cutting-edge technology? This is the promise of synthetic photosynthesis. It’s a revolutionary approach that enhances and mimics natural photosynthesis using both biological and synthetic components.
Synthetic photosynthesis involves creating systems that convert sunlight, water molecules, and carbon dioxide into oxygen and energy-rich compounds. By leveraging innovative engineering and biological processes, synthetic photosynthesis aims to produce clean energy and reduce greenhouse gases. It offers a transformative solution to some of our most pressing environmental challenges.
Global warming represents one of the great existential threats facing us today. Mitigating its worst effects will require the development and implementation of clean energy. A few weeks ago, I discussed microbial energy conversion as a means to this end. Synthetic photosynthesis represents another avenue. In this post, I will explore the science, potential benefits, and challenges of this groundbreaking technology.
Understanding Synthetic Photosynthesis
What is Synthetic Photosynthesis and How Does it Differ From Natural Photosynthesis?
The basic function of photosynthesis is to convert sunlight, water molecules, and carbon dioxide into oxygen and energy-rich compounds. In the case of natural photosynthesis these compounds are sugar molecules. Synthetic photosynthesis harnesses this process to produce hydrogen, methane, or other liquid fuels.
While natural photosynthesis occurs in green plants, algae, and some bacteria, synthetic photosynthesis occurs in artificial systems. The aim of this technology is to produce clean energy and reduce greenhouse gases more efficiently than natural photosynthesis alone. This is done by leveraging advancements in genetic modification, synthetic biology, and materials science. The table below provides a side by side comparison of natural and synthetic photosynthesis.
| Aspect | Natural Photosynthesis | Synthetic Photosynthesis |
| Components | Uses chlorophyll and other biological molecules within chloroplasts to capture light energy and convert it into chemical energy. | Uses biological components, such as genetically modified organisms, and/or advanced materials like semiconductors and catalysts to optimize light absorption and energy conversion. |
| Efficiency | Converts sunlight into chemical energy with an efficiency of about 1-2%. | Efficiency varies with technology. Genetically modified cyanobacteria can achieve solar-to-fuel efficiencies of around 5-10%. Current photoelectrochemical cell systems reach solar-to-hydrogen conversion efficiencies of about 10-19%. Long-term goals aim for efficiencies of 40% or higher. |
| Flexibility | Primarily produces glucose and oxygen, which are used by the plant for growth and metabolism. | Can be designed to produce a variety of fuels and chemicals tailored to specific needs, such as hydrogen for fuel cells or methanol for industrial use. |
| Scalability | Limited by the growth rate and area of plants. | Can be scaled up more easily through engineered systems, allowing for large-scale production of energy and carbon capture on an industrial level. |
Distinction Between Synthetic photosynthesis and Artificial Photosynthesis
You may have heard about Artificial Photosynthesis. How does this differ from Synthetic Photosynthesis. Artificial photosynthesis systems typically refer to setups that use entirely engineered materials to mimic the process of natural photosynthesis. These systems often rely on advanced semiconductors, catalysts, and other non-biological components to convert sunlight, water, and carbon dioxide into fuels and chemicals.
Synthetic photosynthesis on the other hand is a broader term that includes both biological and “bio-hybrid” systems in addition to non-biological (artificial) ones. Bio-hybrid systems integrate biological elements, like enzymes or genetically modified organisms, with synthetic materials. This approach combines the strengths of natural and engineered processes. The goal is to create more efficient and versatile solutions for clean energy production and carbon capture.
The Science Behind Synthetic Photosynthesis
Artificial Systems Mimicking Natural Processes
Artificial systems aim to replicate and enhance the process of photosynthesis using engineered components. They seek to do so with higher efficiency and scalability, addressing the need for renewable energy sources and carbon capture.
Types of Materials Used: Catalysts, Semiconductors
Completely artificial photosynthetic systems leverage advanced materials science to optimize the conversion of light energy into chemical energy. Two critical components are molecular catalysts and semiconductors:
Catalysts: Catalysts play a crucial role in speeding up the chemical reactions involved in synthetic photosynthesis. They facilitate the splitting of water molecules and the reduction of carbon dioxide. Common catalysts used in synthetic photosynthesis include metal oxides like titanium dioxide (TiO₂) and iron oxide (Fe₂O₃). Researchers also use transition metal complexes such as platinum, palladium, and ruthenium due to their high catalytic activity. However, they are exploring more cost-effective and abundant alternatives like nickel and cobalt.
Semiconductors: Semiconductors are essential for absorbing sunlight and generating the free electrons and holes needed for the redox reactions in synthetic photosynthesis. They act similarly to chlorophyll in natural photosynthesis. Researchers commonly use silicon (Si) as a semiconductor in solar panels due to its excellent light-absorbing properties. They also utilize materials like cadmium sulfide (CdS), cadmium selenide (CdSe), and bismuth vanadate (BiVO₄) for their ability to efficiently absorb visible light and convert it into electrical energy.
Methods for Capturing Carbon Dioxide and Producing Oxygen and Energy
Synthetic photosynthesis systems capture carbon dioxide from the atmosphere or from industrial emissions and convert it into valuable fuels and chemicals. This process involves several steps:
Capturing Carbon Dioxide: Specialized materials are used to absorb and concentrate carbon dioxide for use in chemical reactions. Methods include adsorption using metal-organic frameworks or zeolites, which have high surface areas and can selectively capture carbon dioxide molecules. Direct air capture techniques are also employed, pulling carbon dioxide directly from the air using chemical solutions or solid sorbents.
Water Splitting: The process begins with the splitting of water molecules into oxygen, protons, and electrons using sunlight and catalysts. This generates oxygen as a byproduct and provides the necessary protons and electrons for subsequent reactions.
Carbon Dioxide Reduction: The protons and electrons produced from water splitting can then be used to reduce carbon dioxide into various fuels such as methane or methanol. This is achieved through photoelectrochemical cells or other catalytic processes. For instance, in photoelectrochemical cells, sunlight excites the semiconductor material, generating electron-hole pairs that drive the redox reactions needed to produce these fuels.
Engineering Biological Systems
Introduction to Bio-Hybrid Systems that Integrate Biological Components
The term synthetic photosynthesis also refers to systems that integrate biological components to enhance efficiency and functionality. Bio-hybrid systems combine natural biological elements, such as enzymes or genetically modified organisms, with engineered materials to create more effective and versatile photosynthetic processes. These systems leverage the strengths of both biological and synthetic components, aiming to optimize the conversion of sunlight into chemical energy and improve carbon capture.
Genetic Modification of Organisms to Enhance Photosynthesis Efficiency
One approach in synthetic photosynthesis is genetically modifying organisms to boost their photosynthetic capabilities. Scientists can alter the genetic makeup of plants, algae, and bacteria to capture light and convert it into energy more efficiently. For example, they can introduce genes that increase chlorophyll production or enhance the Calvin cycle’s efficiency. This results in higher biomass production and better carbon fixation.
Genetic modification can also make these organisms more resilient to environmental stressors like high temperatures or low light. By creating more robust photosynthetic organisms, scientists aim to develop systems that operate efficiently in various environments. This expands the applicability of synthetic photosynthesis.
Use of Synthetic Biology to Create New Photosynthetic Pathways
Synthetic biology offers exciting opportunities to create entirely new photosynthetic pathways that don’t exist in nature. Researchers can design and assemble novel genetic circuits to develop organisms capable of enhanced or entirely new photosynthetic processes. This may involve introducing non-natural enzymes, bypassing inefficient steps in natural photosynthesis, or creating new metabolic routes to produce desired compounds directly.
For example, scientists can engineer microorganisms to use synthetic pathways that convert carbon dioxide and sunlight into specific fuels or chemicals. These engineered organisms can be designed to produce higher yields of biofuels, like ethanol or butanol, or valuable industrial chemicals. By tailoring these pathways to specific applications, synthetic biology enables the development of highly efficient and customizable photosynthetic systems.
Applications and Potential Benefits of Synthetic Photosynthesis
Carbon Capture and Clean Energy Via Synthetic Photosynthesis

Synthetic photosynthesis offers an effective solution for capturing and reducing atmospheric carbon dioxide (CO₂). These systems utilize advanced materials and catalysts to enhance the efficiency of CO₂ capture and conversion. For instance, metal-organic frameworks and other porous materials can selectively absorb CO₂ molecules. Once captured, the CO₂ undergoes sunlight-driven chemical reactions to produce a range of carbon-neutral compounds, including fuels like methane and methanol, as well as other useful hydrocarbons such as ethylene and formic acid. Ethylene is a valuable raw material in the production of plastics, while formic acid can be used in various chemical processes and as a preservative.
Additionally, synthetic photosynthesis can split water into hydrogen and oxygen, producing hydrogen fuel as a clean fuel. This hydrogen can be used in fuel cells for electric vehicles, power generation, and other industrial applications. Since hydrogen combustion only emits water, it offers a zero-emission energy source. Furthermore, the production of hydrogen through photoelectrochemical cells utilizes hydrogen atoms to enhance energy-dense fuels.
The carbon-neutral nature of the fuels and chemicals produced through synthetic photosynthesis means they do not contribute new CO₂ to the atmosphere. This makes synthetic photosynthesis a key player in the shift away from fossil fuels, helping to reduce our carbon footprint and support a more sustainable energy future. Furthermore, natural gas can be synthesized via carbon dioxide reduction processes, providing an alternative to traditional fossil-derived natural gas sources.
Integration with Existing Renewable Energy Technologies
Synthetic photosynthesis can work alongside other renewable energy technologies to create a more resilient and efficient energy system. While solar photovoltaic (PV) panels and wind turbines provide renewable electricity, they often face challenges with intermittency and energy storage. Since synthetic photosynthesis uses solar energy to produce hydrogen and other fuels, it provides a way to store energy in chemical form.
This process can be integrated with existing renewable infrastructure. For instance, during periods of excess renewable electricity production—such as sunny days or windy conditions—synthetic photosynthesis energy conversion systems can use the surplus electricity to produce hydrogen. This hydrogen can then be stored and later converted back into electricity when renewable energy production is low, ensuring a stable and continuous energy supply. This approach not only maximizes the use of renewable energy but also reduces the need for fossil fuel-based backup power plants.
Furthermore, the hydrogen and other fuels produced through synthetic photosynthesis have versatile applications beyond power generation. They can be used as clean fuels for transportation, or for industrial processes like steel and cement production, which are traditionally challenging to decarbonize. By creating a carbon-neutral fuel source, synthetic photosynthesis extends the benefits of renewable energy across various sectors.
Agricultural Applications of Synthetic Photosynthesis
Synthetic photosynthesis offers numerous advantages for agriculture, providing innovative solutions to enhance crop productivity and sustainability. One key benefit is that the hydrogen it produces can be used to create ammonia for fertilizers. Not only is this process cleaner than traditional methods, but producing fertilizers locally reduces transport costs and energy consumption.
Additionally, synthetic photosynthesis can significantly reduce water consumption. Traditional fertilizer production methods are much more water-intensive. Moreover, while synthetic photosynthesis does use water to produce hydrogen, it can utilize non-potable water sources, such as brackish water. This conserves freshwater for irrigation and other essential uses.
Overall, synthetic photosynthesis holds significant promise for enhancing food security. By providing additional energy, water, and nutrients to crops, synthetic systems can help address the challenges of modern agriculture and ensure a stable, sustainable food supply for the future. As research and development in this field continue to advance, the integration of synthetic photosynthesis into agricultural practices could play a crucial role in feeding a growing global population.
Cleaner Chemical Production Methods

U.S. Department of Energy from United States, Public domain, via Wikimedia Commons
Synthetic photosynthesis is not limited to energy production; it also has significant potential in the industrial sector for producing valuable chemicals and materials. By converting carbon dioxide and sunlight into specific chemical compounds, synthetic photosynthesis can create raw materials for various industries. These compounds can be used to manufacture plastics, pharmaceuticals, and other essential products.
For example, the process can be tailored to produce methanol, a versatile chemical used as a fuel, solvent, and antifreeze. Methanol can also serve as a building block for creating more complex chemicals. Similarly, synthetic photosynthesis can generate ethylene, a key ingredient in the production of polyethylene, the most common plastic. This ability to produce valuable chemicals from renewable resources reduces dependence on fossil fuels and supports the transition to a sustainable industrial economy.
In addition, synthetic photosynthesis can assist in managing industrial waste. By converting waste gases into valuable products, this technology can reduce the environmental impact of manufacturing processes. This not only helps in reducing pollution but also creates a circular economy where waste is transformed into resources. As research progresses, the potential applications of synthetic photosynthesis will continue to expand, underscoring its importance in addressing both industrial and environmental challenges.
Current Research and Development Projects in Synthetic Photosynthesis Technology
Harvard University’s Artificial Leaf
Under Dr. Daniel Nocera’s leadership, Harvard University developed the artificial leaf, an artificial photosynthesis device. This silicon chip is coated with water-splitting catalysts that mimic natural photosynthesis. It uses sunlight to split water molecules into hydrogen fuel and oxygen atoms.
The artificial leaf produces clean fuel stored and used on-site in fuel cells. Remarkably, it can split any type of water, from seawater to puddles, valuable in regions with limited fresh water. While natural photosynthesis captures only 1% of the sun’s energy, Nocera’s artificial leaf utilizes nearly 10% thanks to a silicon-germanium material enhancing light absorption across the full solar spectrum.
The Bionic Leaf and Beyond
Building on the success of the artificial leaf, Nocera’s team engineered the “bionic leaf” to convert hydrogen into liquid biofuels. Using an engineered bacterium, the system combines hydrogen fuel with carbon dioxide to create long-chain hydrocarbons. This results in biomass that grows ten times faster than natural organisms and can store sunlight indefinitely.
The bionic leaf produces liquid fuels at efficiencies 100 to 1,000 times greater than traditional biofuels. Additionally, they developed a cyanobacterium, Xanthobacter autotrophicus, that produces ammonia by combining hydrogen with nitrogen, reducing the environmental impact of ammonia fertilizer production.
Liquid Sunlight Alliance – Caltech
Professor Harry Atwater leads the Liquid Sunlight Alliance at Caltech, a Department of Energy Solar Fuels Hub project focused on advancing artificial photosynthesis systems. This initiative aims to develop systems capable of converting sunlight into chemical fuels, such as hydrogen, through photoelectrochemical processes.
The project emphasizes integrating advanced semiconductors and catalysts to enhance the efficiency of solar-to-fuel conversion. Recent advancements have demonstrated solar-to-hydrogen efficiencies exceeding 19%, utilizing innovative designs like transparent catalysts and protective coatings to improve device stability and performance. The Liquid Sunlight Alliance seeks to create sustainable energy production solutions, contributing to a circular carbon economy by balancing hydrocarbon fuel use with carbon capture and conversion.
University of Warwick – Synthetic Photosynthesis for Space Exploration
Assistant Professor Katharina Brinkert at the University of Warwick is leading research on artificial photosynthesis for space exploration, aiming to produce oxygen and fuel on the Moon and Mars. These photoelectrochemical (PEC) systems mimic natural photosynthesis, using semiconductor materials with metallic catalysts to convert sunlight, carbon dioxide, and water into oxygen and hydrogen without electricity.
This technology is ideal for long-duration missions due to its reduced volume, weight, and maintenance needs. Additionally, the systems can operate efficiently in Mars’ lower light conditions by using solar mirrors to concentrate sunlight. This innovative research not only advances sustainable life support in space but also offers potential green energy solutions for Earth.
Innovations in Synthetic Photosynthesis Output from the University of Chicago and the University of Michigan
Researchers at the University of Chicago, led by chemistry professor Wenbin Lin, have developed a groundbreaking artificial photosynthesis system that outperforms previous efforts by an order of magnitude. This innovative approach converts sunlight, carbon dioxide, and water into methane fuel, a highly concentrated energy source.The team employed a metal-organic framework (MOF) combined with amino acids to enhance the efficiency of both water splitting and carbon dioxide reduction, key processes in artificial photosynthesis. Although the technology requires significant scaling to meet global energy demands, it holds promise for producing methane fuel and other valuable chemicals, such as pharmaceuticals and nylons, more sustainably.
Meanwhile, University of Michigan researchers have developed a cutting-edge artificial photosynthesis system that efficiently produces ethylene. By linking carbon atoms together, the system synthesizes ethylene from carbon dioxide with unprecedented efficiency and stability. This breakthrough could revolutionize the sustainable production of plastics and potentially pave the way for generating liquid fuels, offering greener alternatives to traditional manufacturing processes.
Lawrence Berkeley National Laboratory – Artificial Leaf Technology
Dr. Peidong Yang at Lawrence Berkeley National Laboratory is leading the development of artificial leaf technology that emulates natural photosynthesis. This system combines semiconductor nanowires with biological components to capture sunlight and convert carbon dioxide and water into chemical fuels, such as hydrogen.
By integrating synthetic and biological elements, the project aims to create scalable solutions for clean energy production. The artificial leaf system has shown potential for solar-to-fuel conversion, with ongoing research focused on enhancing efficiency and producing value-added chemicals.
Universidad Ana G. Méndez – Organic Mediators for Synthetic Photosynthesis
The Nanomaterials Research Group at Universidad Ana G. Méndez, including María Cotto and José Ducongé, is developing new organic mediators to improve the stability and performance of artificial photosynthesis systems. Their work aims to enhance the efficiency of photo-electrochemical cells in converting sunlight into usable energy, potentially leading to more effective carbon-based fuel production.
Max Planck Institute for Terrestrial Microbiology – Synthetic Pathways for Enhanced Photosynthesis
Professor Tobias Erb at the Max Planck Institute for Terrestrial Microbiology leads research on enhancing photosynthetic systems through synthetic biology. His team developed artificial chloroplasts by combining natural chloroplast membranes with a synthetic enzymatic module called the CETCH cycle. The CETCH cycle consists of 18 biocatalysts that convert carbon dioxide more efficiently than natural processes.
This synthetic pathway fixes carbon up to 100 times more efficiently than previous methods. Using microfluidic technology to create cell-sized droplets, the research highlights synthetic biology’s potential in developing autonomous photosynthetic systems. This work opens possibilities for sustainable biofuel production and large-scale chemical manufacturing.
Joint Center Initiative
The Joint Center for Artificial Photosynthesis (JCAP), operated from 2010 to 2021. It had two main centers are located at the California Institute of Technology and the Lawrence Berkeley National Laboratory in addition to a number of other institutes, including the University of California and Stanford. It focused on integrating molecular catalysts with semiconductors to enhance light energy utilization and charge separation. During their 10-year run, JCAP scientists have set new records for artificial photosynthesis by increasing solar-to-chemical energy efficiency performance from less than 1 percent to 19 percent and by designing highly stable solar-fuels generators.
Synthetic Photosynthesis Technical Challenges
Synthetic photosynthesis Efficiency and Scalability Issues
One of the main technical challenges facing synthetic photosynthesis is achieving high efficiency. While synthetic systems have made significant progress, they still face hurdles in maximizing light absorption and converting it into chemical energy. Current systems do not yet match the high efficiencies seen in traditional solar cells. Improving these conversion rates is crucial for making synthetic photosynthesis a viable large-scale solution.
Scalability is another significant challenge. Laboratory experiments often show promising results, but scaling these systems to industrial levels is complex. Large-scale synthetic photosynthesis systems require substantial infrastructure, including extensive arrays of photoelectrochemical cells and large surface areas to capture sufficient sunlight. Ensuring these systems can be efficiently and economically scaled to meet global energy demands is essential for their widespread adoption.
Durability and Cost of Materials
The durability of materials used in synthetic photosynthesis systems presents another critical challenge. Many current materials, such as catalysts and semiconductors, degrade over time, especially when exposed to harsh environmental conditions like high temperatures and intense sunlight. This degradation can lead to decreased efficiency and frequent replacements, making the systems less viable for long-term use.
Cost is closely tied to durability. High-performance materials that are stable and efficient often come with a high price tag. For example, precious metals like platinum and ruthenium are excellent catalysts but are expensive materials and scarce. Finding cost-effective alternatives that do not compromise performance is vital for the widespread adoption of synthetic photosynthesis.
Researchers are exploring various approaches to address these issues. Developing new materials that are both durable and affordable is a key focus. For instance, using earth-abundant materials like iron and copper as catalysts is an area of active research. Additionally, advancements in nanotechnology and materials science could lead to the creation of more resilient and efficient components.
Environmental Impact of Large-Scale Implementation
While synthetic photosynthesis offers significant environmental benefits, large-scale implementation raises several environmental concerns. Producing and deploying synthetic photosynthesis systems require substantial resources and land. If not managed carefully, this could lead to habitat disruption and biodiversity loss.
Additionally, the materials used in synthetic photosynthesis systems, such as certain catalysts and semiconductors, may have environmental impacts if not properly disposed of or recycled. Extracting and processing raw materials for these components can result in pollution and resource depletion. Ensuring that the lifecycle of these materials is sustainable and environmentally friendly is crucial for minimizing negative impacts.
Moreover, the carbon dioxide reduction processes involved in synthetic photosynthesis must be carefully managed to ensure they contribute positively to carbon neutrality. Utilizing metal-organic frameworks and other porous materials can enhance the carbon source conversion efficiency, but their production and disposal must adhere to green energy solutions standards to prevent unintended environmental harm.
Additionally, the process of artificial photosynthesis needs to consider the impact of carbon atoms and carbon monoxide emissions. Integrating nicotinamide adenine dinucleotide phosphate (NADPH) into light-independent reactions can optimize the chemical process without exceeding energy needs. This holistic approach ensures that synthetic photosynthesis not only addresses human needs for clean energy but also aligns with environmental sustainability.
Emerging Technologies
Novel Catalysts
The field of synthetic photosynthesis is rapidly evolving, with researchers continually developing new materials and methods to enhance efficiency and scalability. One promising area of development is the use of novel catalysts. Researchers are focusing on earth-abundant and cost-effective materials, such as iron, nickel, and cobalt, to replace expensive materials and rare elements like platinum and ruthenium. These new catalysts aim to maintain high performance while significantly reducing costs and improving accessibility.
Advanced Nanomaterials
In addition to new catalysts, advanced nanomaterials are being explored to optimize light absorption and energy conversion. Materials like graphene and carbon nanotubes are showing promise in improving the efficiency of photoelectrochemical cells. These nanomaterials can provide a larger maximum surface area for chemical reactions and enhance the conductivity of the cells, leading to better overall performance.
Bio-Inspired Designs
Another emerging method is the use of bio-inspired designs. By studying the structure and function of natural photosynthesis in green plants and other food-producing organisms, scientists are creating synthetic systems that mimic these efficient processes. For example, researchers are developing artificial leaves that replicate the hierarchical structure of plant leaves, allowing for more efficient light capture and water splitting.
Predictions for the Next Decade of Synthetic Photosynthesis Research
Improving Efficiency
Over the next decade, synthetic photosynthesis research is expected to make significant strides, leading to more practical and widespread applications. A key area of focus will be improving the overall efficiency of these systems. As new materials and methods are developed, we anticipate substantial increases in the conversion rates of sunlight to chemical energy, bringing these systems closer to commercial viability. Researchers are particularly focused on optimizing catalysts and light-absorbing semiconductors to enhance performance.
Moreover, advancements in understanding the roles of adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) in the process of photosynthesis provide insights into optimizing light-dependent reactions within artificial photosynthesis systems.
Addressing Scalability
Scaling synthetic photosynthesis to meet global energy demands requires setups that can produce energy-dense fuels at a large scale. Collaborative efforts with the U.S. Department of Energy and other research teams are crucial for securing the necessary funding and resources to advance these technologies. Efforts are focused on efficient, cost-effective production methods.
One promising approach uses semiconducting microwire arrays with flexible polymeric membranes for scalable solar fuel generation. Advances in nanotechnology have led to earth-abundant electrocatalysts and materials that stabilize light absorbers, improving efficiency and durability. Integrating artificial photosynthetic systems into existing energy infrastructure is also being explored for large-scale deployment, aiming to create robust systems that maintain high performance, making synthetic photosynthesis a viable global energy solution.
Furthermore, innovations in metal ions and transition metal complexes will enhance the catalytic activity and durability of synthetic photosynthesis systems. This promises to them more viable for large-scale deployment.
Integration with Other Renewable Energy Technologies
Integration with other renewable energy technologies is another promising direction. Researchers are exploring ways to combine synthetic photosynthesis with solar PV, wind turbines, and energy storage systems to create more resilient and efficient energy grids. This hybrid approach could help address the intermittency issues of renewable energy sources, ensuring a steady and reliable supply of clean energy.
Additionally, integrating synthetic photosynthesis with photovoltaic cells and solar power systems can enhance overall light absorption and energy conversion efficiency. By utilizing single layer semiconductors and metal-organic frameworks, these integrated systems can maximize the capture of light energy and convert it into hydrogen fuel and other chemical products.
Collaborative Efforts
The next decade will likely see increased collaboration between academia, industry, and governments. Public and private partnerships will be essential for advancing research, securing funding, and developing regulatory frameworks that support the deployment of synthetic photosynthesis technologies. Such collaborations will help accelerate the transition from laboratory research to real-world applications.
Conclusion

A Promising Solution to Global Challenges
Synthetic photosynthesis represents a promising solution to some of the world’s most pressing challenges. By mimicking and enhancing natural photosynthesis, this technology captures carbon dioxide and produces clean energy, offering a dual benefit for climate change mitigation and renewable energy production. I’ve explored how synthetic photosynthesis works, its current research advancements, and its potential applications in carbon capture, renewable energy, agriculture, and industrial processes.
Bright Future and Ongoing Innovations
Looking ahead, the future of synthetic photosynthesis is bright. Ongoing research continues to push the boundaries of efficiency and scalability. Innovations in materials and interdisciplinary collaborations are key to overcoming existing challenges. As technology advances, synthetic photosynthesis could play a pivotal role in reducing our reliance on fossil fuels, lowering greenhouse gas emissions, and creating sustainable energy solutions. Additionally, its integration into various sectors, from agriculture to industry, underscores its versatility and far-reaching impact.
Enhancing Energy Production and the Circular Carbon Economy
Synthetic photosynthesis not only enhances clean energy production but also contributes to the development of energy-dense fuels. By leveraging molecular catalysts and semiconductors, these systems optimize light absorption and energy conversion, making them more efficient than traditional solar cells. The ability to produce hydrogen fuel and other liquid fuels from carbon dioxide reduction processes highlights the technology’s potential to support a circular carbon economy.
Synergy with Other Renewable Energy Technologies
Moreover, synthetic photosynthesis aligns with other renewable energy technologies like solar power and wind turbines, enhancing the overall resilience and efficiency of our energy systems. The production of hydrogen through photoelectrochemical cells and the use of metal-organic frameworks exemplify the innovative approaches driving this field forward. As research teams and institutions continue to make innovations, the ultimate goal of achieving efficient artificial photosynthetic systems becomes increasingly attainable.
Your Thoughts
I’d love to hear your opinions on synthetic photosynthesis and its potential impact on our future. What do you think are the biggest challenges or opportunities in this field? Please share your thoughts and questions in the comments below!
Be sure to visit bleedingedgebiology.com next week for another “bleeding edge” topic!
Additional Materials on Synthetic Photosynthesis
To further explore synthetic photosynthesis, here are some recommended books, articles, TED Talks, and videos/documentaries.
Articles
- “Principles and Applications of Artificial Photosynthesis” By Shunichi Fukuzumi Royal Society of Chemistry (2023) This book aims to provide a unified view, and future perspective, of artificial photosynthesis while discussing and reviewing all of the artificial molecular processes together.
- “Microbial Photosynthesis: From Basic Biology to Artificial Cell Factories and Industrial Applications” by Rachapudi V. Sreeharsha and S. Venkata Mohan Springer (2024). This book uncovers the basic principles of microbial photosynthesis and the latest technological interventions of this crucial phenomenon.
- “Taming the Sun: Innovations to Harness Solar Energy and Power the Planet” by Varun Sivaram MIT Press (2018) in Energy expert Varun Sivaram warns that the world is not yet equipped to harness erratic sunshine to meet most of its energy needs. Innovation can brighten those prospects, Sivaram explains, drawing on firsthand experience and original research spanning science, business, and government.
- Kubis, A., & Bar-Even, A. (2019). “Synthetic biology approaches for improving photosynthesis” Nature Reviews Molecular Cell Biology, 20(1), 1-15. This review discusses various synthetic biology techniques aimed at enhancing photosynthesis efficiency. It highlights strategies such as engineering Rubisco, optimizing the Calvin cycle, and introducing carbon-concentrating mechanisms to improve carbon fixation in plants.
- Brinkert, K (2023) “Space colonies: how artificial photosynthesis may be key to sustained life beyond Earth” The Conversation. Recent advances in making artificial photosynthesis may well be key to surviving and thriving away from Earth.
- Sivaram, V. (2018) “The race to invent the artificial leaf” MIT Technology Review. In this excerpt from his book Taming the Sun, Varun Sivaram follows the research paths of two rival scientists determined to find a way to wring fuel out of thin air.
TED Talks/Documentaries/Videos
- “Towards Artificial Photosynthesis” by Alexey Cherevan TEDxTUWienSalon 2020. Alexey Cherevan focuses on the idea of artificial photosynthesis. He discusses the capabilities and complexity of natural photosynthesis and shows how material scientists can be inspired by nature to recreate the process.
- “How green hydrogen could end the fossil fuel era” by Vaitea Cowan TED 2022. As climate change accelerates, finding clean alternatives to fossil fuels is more urgent than ever. Social entrepreneur Vaitea Cowan believes green hydrogen is the answer. Watch as she shares her team’s work mass producing electrolyzers — devices that separate water into its molecular components: hydrogen and oxygen — and shows how they could help make green, carbon-free fuel affordable and accessible for everyone.
- “Can we hack photosynthesis to feed the world?” by Steve Long TED 2023. Photosynthesis is one of the most important processes on the planet, helping produce the food we eat and the air we breathe. Crop scientist Steve Long thinks it could be more efficient — and he’s intent on giving it a boost. He shows how hacking photosynthesis could help feed the world all while reducing climate change.
- “Fuels and Food from Sunlight, Air and Water” by Daniel Nocera MoleCluesTV 2017 Nocera explores the challenges of creating a fuel from hydrogen and carbon dioxide and discusses his plans to create fuels and fertilizers using sunlight, air, and water.
- “The Artificial Leaf” by Jared P. Scott & Kelly Nyks The Artificial Leaf is a 2nd Place Winner in the $200,000 GE Focus Forward Filmmaker Competition. Inspiring short film about Dan Nocera’s simple formula to save the planet: sunlight + water = energy for the world.
- “Photosynthesis: Crash Course Biology” – CrashCourse (April 23). This educational video explains the extremely complex series of reactions whereby plants feed themselves on sunlight, carbon dioxide and water, and also create some by products we’re pretty fond of as well.
- “Breaking the limits of natural photosynthesis with synthetic biology” By Tobias Erb TU Dresden. Tobias Erb is synthetic biologist and Director at the Max Planck Institute for terrestrial Microbiology in Marburg, Germany. Research in Erb’s lab crosses multiple scales: from the molecular mechanisms of carboxylases to their ecological relevance, and from understanding the evolution of natural CO2-fixation to developing new-to-nature solutions, such as synthetic CO2-fixation pathways and artificial chloroplasts.


